Advanced solution in Thermodynamics

The Thermodynamic Database
|
Advanced solution in Thermodynamics |
|
 |
|
|
The Thermodynamic Database |
Creation of a Project Concept and Process Analysis
based on thermodynamic calculations and measurements
1.Process concept
| In an experimental furnace, CuFeS2 is oxidized at 1250 °C. The goal is to form a matte phase consisting primarily of Cu and Cu2S,
CuFeS2 from the input material dissolves in the matte phase. O2 from air jets reacts with CuFeS2, FeS, and Cu2S, mainly in the matte phase.
|
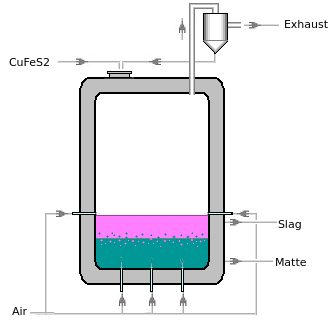 |
2. Estimation of the process flow based on thermodynamic calculations
Using thermodynamic calculations, we determine the amount of O2 that must react with the input material CuFeS2 in the furnace to ensure that the maximum amount of copper exists in the matte phase. The figure right shows the calculation results for the use CuFeS2 of 40
[kg/h] The O2 amount of 17.5 [kg/h] corresponds to an air flow rate of 58.3 [Nm³/h]. If this amount of O2 is converted in the furnace, no O2 can be detected in the exhaust gas.
|
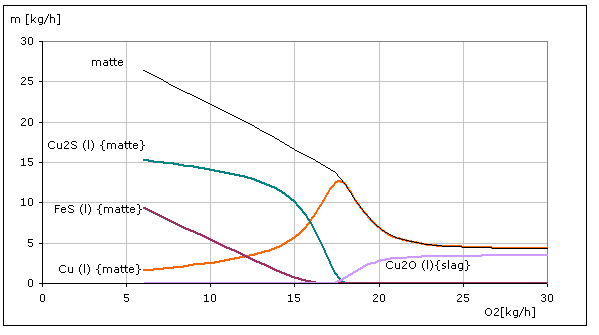 |
| As can
be seen from the measurements at the almost steady state of the process,
the maximum Cu(l)-formation is achieved, when air flow rate is 72 [Nm³/h]. This means O2-Flowrate is 21.3 [kg/h]. Same time, O2 concentration in exhaust is measured 4.1%.
The amount of the expected leakage air is probably
insignificant in the exhaust, It is more likely that
an O2 amount of 3.8 [kg/h] (3.8=21.3-7.5 ) |
|
4. Comparison of measurement results with thermodynamic calculations
The following tables show the elemental composition of the slag according to the thermodynamic calculation and the range of the measured values.| Slag Mass: calculation 103[kg], measured ~100[kg]
|
Matte Mass: calculation 13.6[kg], measured 12 ~ 14[kg]
|
|||||||||||||||||||||||||||||
5. Summary
Through thermodynamic calculations, we can easily estimate the reactions and
product composition in high-temperature reactors quite accurately.
For a process analysis, it is very helpful if the substantial composition
of the input materials is known.
This allows us to estimate which proportion of the gases used in the reactor
react.
The substantial composition of the input material must also be known for a
reliable heat balance.
We often only have the data of the elemental composition of an input
material.
The substantial composition of a material can also be determined by
thermodynamic calculations (sometimes only roughly but often quite
accurately).
Some methods known to
date for determining the substantial composition based on the elemental
analysis of a material.
Often a simple stoichiometric calculation helps us to determine the
substantial composition of a materials.