Advanced solution in Thermodynamics

Datasets of Aqueous Substances
|
Advanced solution in Thermodynamics |
|
|
|
|
|
Datasets of Aqueous Substances |
5.6. Thermodynamic systems with aqueous Substances
5.6.1. Calculation of the solubility of substances in water based on the datasets
The datasets of the aqueous substances, like AgCl[aq], AgNO3[aq], NaCO3[aq], CaCO3[aq] etc.
valid for the sum of the all parts of the substance in the aqueous solution.
AgNO3[aq] contains all parts of the solved AgNO3 in water:
AgNO30 , Ag+
and NO3-
mAgNO3[aq] = mAgNO3°+ mAg++ mNO3-
You can calculate also the solubility of a substance in water using the datasets of the aqueous substances.
For the solution equation
AgNO3 (s) = AgNO3[aq]
the equilibrium constant K is defined with
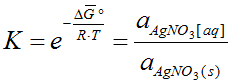
When AgNO3 exists as pure substance in the equilibrium state, then activity is one
![]()
At high concentrations we can write activity a nearly equal to mol fraction x
![]()
Equilibrium constant K is nearly equal to the mol fraction x of AgNO3[aq]
![]()
5.6.1.1. AgNO3 Solubility in Water
In the following graphic, the line shows the solubility of AgNO3 in water [g
AgNO3/100 g water] in depend of
the temperature,
which is calculated using AsTher Process Calculator for MS Excel und measured
values given in different sources
Ref.:
Line AgNO3[aq]: Calculation result from AsTher Thermodynamic Database
[CRC] : CRC Handbook of Chemistry and Physics; CRC Press LLC 2004
[Perry]: Robert H. Perry; Cecil H. Chilton: Chemical Engineers’ Handbook; McGraw Hill, 1973
AgNO3 (s) = AgNO3[aq] (l)
| T [ C ] | dH [J/mol] | dS [J/mol K] | dG [J/mol] | K | T dS [J/mol] | dCp [J/mol K] |
| 0 | 22590.3 | 65.06 | 4818.63 | 0.11982 | 17771.6 | -0.00781 |
| 10 | 22590.2 | 64.25 | 4397.57 | 0.154437 | 18192.6 | -0.00588 |
| 20 | 22590.2 | 63.36 | 4016.11 | 0.192483 | 18574.1 | -0.00395 |
| 30 | 22590.1 | 62.4 | 3673.82 | 0.232797 | 18916.3 | -0.00202 |
| 40 | 22590.1 | 61.38 | 3370.28 | 0.274046 | 19219.8 | -0.00009 |
| 50 | 22590.1 | 60.3 | 3105.11 | 0.314835 | 19485 | 0.00184 |
| 60 | 22590.2 | 59.17 | 2877.94 | 0.353808 | 19712.2 | 0.00376 |
| 70 | 22590.2 | 58 | 2688.43 | 0.38973 | 19901.8 | 0.00569 |
| 80 | 22590.3 | 56.79 | 2536.27 | 0.42156 | 20054 | 0.00762 |
| 90 | 22590.4 | 55.54 | 2421.14 | 0.448486 | 20169.2 | 0.00955 |
| 100 | 22590.5 | 54.26 | 2342.77 | 0.469951 | 20247.7 | 0.01148 |
5.6.1.2. State Functions of AgNO3[aq] in AsTher Thermodynamic Database
AgNO3[aq] (l)
| T [ C ] | Cp [J/mol C] | H [J/mol] | S [J/mol K] | G [J/mol] |
| 0 | 88.31 | -104067 | 197.751 | -158083 |
| 10 | 90.21 | -103174 | 200.15 | -159847 |
| 20 | 92.1 | -102263 | 202.423 | -161603 |
| 25 | 93.05 | -101800 | 203.516 | -162478 |
| 30 | 93.99 | -101332 | 204.583 | -163351 |
| 40 | 95.89 | -100383 | 206.64 | -165092 |
| 50 | 97.78 | -99415 | 208.605 | -166825 |
| 60 | 99.67 | -98427 | 210.486 | -168551 |
| 70 | 101.57 | -97421 | 212.29 | -170268 |
| 80 | 103.46 | -96396 | 214.023 | -171978 |
| 90 | 105.35 | -95352 | 215.691 | -173680 |
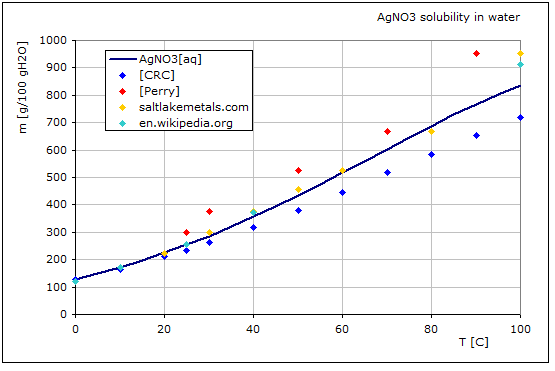
5.6.2.1. Na2SO4 Solubility in Water
Na2SO4[aq] -> Na2SO4 (s)
The solubilty of the Na2SO4 is calculated using datasets in AsTher and shown in the following graphic
Na2SO4[aq] -> Na2SO4 (s)
| T [ C ] | dH [J/mol] | dS [J/mol K] | dG [J/mol] | K | T dS [J/mol] | dCp [J/mol C] |
| 0 | -1688 | -49.187 | 11747 | 0.00567015 | -13436 | 0.0697 |
| 10 | -1688 | -43.160 | 10533 | 0.01140102 | -12221 | 0.0089 |
| 20 | -1688 | -36.821 | 9106 | 0.02385014 | -10794 | -0.0211 |
| 25 | -1688 | -33.542 | 8312 | 0.03497209 | -10001 | -0.0246 |
| 30 | -1688 | -30.195 | 7465 | 0.05172326 | -9154 | -0.0205 |
| 40 | -1683 | -29.398 | 7523 | 0.05561135 | -9206 | 0.0998 |
| 50 | -1682 | -29.538 | 7863 | 0.05358249 | -9545 | 0.0236 |
| 60 | -1682 | -29.632 | 8189 | 0.05199929 | -9872 | -0.0218 |
| 70 | -1683 | -29.678 | 8501 | 0.05080696 | -10184 | -0.0365 |
| 80 | -1683 | -29.679 | 8798 | 0.0499632 | -10481 | -0.0205 |
| 90 | -1683 | -29.636 | 9079 | 0.0494357 | -10762 | 0.0261 |
| 100 | -1682 | -29.550 | 9344 | 0.04920028 | -11027 | 0.1035 |
5.6.2.2. State Functions of Na2SO4[aq]
State functions of Na2SO4[aq] corresponding to the dataset in AsTher
| T [ C ] | Cp [J/mol C] | H [J/mol] | S [J/mol K] | G [J/mol] |
| 0 | 124 | -1392651 | 89.39 | -1417067 |
| 10 | 125 | -1391406 | 99.89 | -1419690 |
| 25 | 128 | -1389504 | 116.05 | -1424106 |
| 20 | 127 | -1390143 | 110.61 | -1422569 |
| 30 | 129 | -1388861 | 121.54 | -1425706 |
| 40 | 131 | -1387557 | 126.55 | -1427187 |
| 50 | 133 | -1386239 | 130.55 | -1428427 |
| 60 | 134 | -1384904 | 134.53 | -1429722 |
| 70 | 136 | -1383552 | 138.48 | -1431072 |
| 80 | 138 | -1382184 | 142.41 | -1432476 |
| 90 | 139 | -1380799 | 146.32 | -1433935 |
| 100 | 141 | -1379397 | 150.21 | -1435449 |
5.6.3. Process Modelling using Datasets of Aqueous Substances
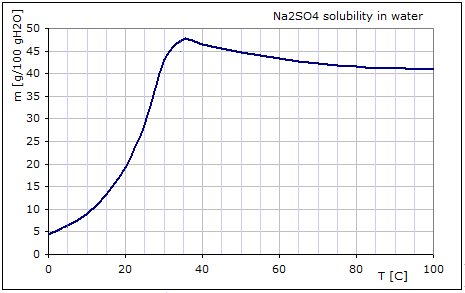
Calculation of the heat- and mass balance of the desulfuration-process of the battery recycling
A directly reduction of the battery paste from recycling causes height
SO2-concentration in exhausts of the furnaces for lead production.
Sometimes the paste is desulfurised using NaOH before the reductions process to lead
production.
In the Desulfuration reactor, PbSO4 and NaOH react at 30-35°C:
PbSO4 (s) + 2 NaOH[aq] -> Na2SO4[aq]+ PbO (s)
NaSO4 is solved in water.
In the Crystallisation reactor, Na2SO4 (s) forms at 5°C:
Na2SO4[aq] -> Na2SO4 (s)
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5.6.4. Important notes for calculations using AsTher Application Equilibrium or Process Calculator
When you define sometimes liquid phase and solid phase as following
Liquids| (l) | In [kg] | Out [kg] | w% | a | a.c. |
| H2O | 603.31609 | 603.56581 | 83.534857 | 0.92307476 | |
| Na2SO4[aq] | 25.30796 | 0.19361783 | 0.02679714 | 3.7543E-05 | |
| NaCl[aq] | 0.00389118 | 0.00035148 | 4.8646E-05 | 1.6561E-07 | |
| NaOH[aq] | 105.77432 | 105.74997 | 14.636032 | 0.07283515 | |
| As2O3[aq] | 2.7464E-05 | 5.9543E-17 | 8.2408E-18 | 8.2938E-21 | |
| Ca(OH)2[aq] | 13.564981 | 13.021939 | 1.8022654 | 0.00484381 |
Solids
| (s) | In [kg] | Out [kg] | w% | a | a.c. |
| Na2SO4 (s) | 24.206453 | 97.980256 | 4.69E-03 | -1 | |
| NaCl (s) | 0 | 0 | 0 | -1 | |
| NaOH (s) | 0 | 0 | 0 | -1 | |
| As2O3 (s) | 0 | 0 | 0 | -1 | |
| Ca(OH)2 (s) | 0 | 0 | 0 | -1 |
then you should enter in the column for Activity coefficient (a.c.)
-1 or (1): The formation of the substances is able, when the substance exist as
pure
substance, not in any mixture of solids.
When only the formation of the pure substances are permitted in calculations, the calculation my be several seconds longer.
When the formation of the substances not possible,
then you can deselect the substance from thermodynamic system or enter for zero
Activity coefficient.
When you do not enter any data for activity coefficient,
then the substance is regarded in a solution or mixture, but not as pure
substance.
5.6.5. References and Accuracy of the
Datasets
The solubility data of the aqueous substances and enthalpy of the solution can
not be always verified more than two different data sources.
We can not say, that the datasets were exact the real physical values.
But we can say, that the datasets can enable a process modelling sufficient to
process design,
like Calculation of the heat- and mass-balance of
the desulfuration-process of the battery recycling
Recommanded Source for thermodynamic data
ANDRA: Sélection de constantes thermodynamiques pour les éléments majeurs, le
plomb et le cadmium, Jui"et 2006
Document Public
Centre scientifique et technique - Service Environnement et Procédés:
A comparation many datasets of the compounds of Al, C, Ca, Cd, Cl, K, Mg,
N, Na, O, P, Pb, S etc. from different sources
the another known data sources
CODATA
NIST
JANAF
CRC (Handbook Chemistry and. Physic)
Thermochimie5
Perry (Chem. Eng. Handbook)
webserver.dmt.upm.es
intechopen.com
en.wikipedia.org