Advanced solution inThermodynamics

Thermodynamic Datenbase und Process Design
|
Advanced solution inThermodynamics |
|
 |
|
|
Thermodynamic Datenbase und Process Design |
Thermodynamic calculation and Process modelling
Content
1. Calculation of an equilibrium state in AsTher
2. Answer to the questions whether and how a process
can be modelled or represented using thermodynamic calculations.
Examples and explanations
An example shows how a process concept is
created and a process analysis is carried out using thermodynamic calculations.
Determination of substantial
composition based on elementary analysis of a material
1. Calculation of an equilibrium state in AsTher
The initial state for calculating an equilibrium state is a hypothetical mixture
consisting of the selected substances in all states of matter: gas, liquid,
solid, and plasma.
The Lagrange coefficients of the elements in the equilibrium state are
determined iteratively according to the phases, ensuring an elemental balance
between the input composition and the calculated composition.
The extended option "Turbulent System: Heigh Entropy"
can be selected in the application
Equilibrium (Equlibrm.exe) and
Process Calculator (XProCalc.exe)
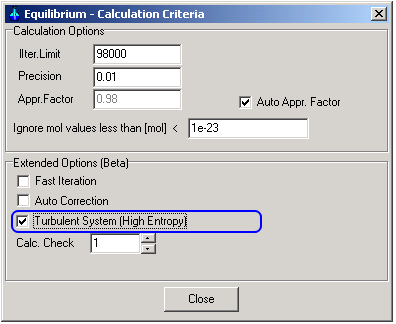
1.1. Selecting the calculation option "Turbulent System (High Entropy)"
the calculation is terminated when,
the given pressure is reached in the gas phase,
or
when the sum of the activities of the substances in a phase corresponds to the
system pressure.
The sum of the chemical activity of substances: is not in each phase
Σa i
≈ 1
The calculation algorithm using "High Entropy" has no effect on the
calculation results,
if the reaction system consists of only one phase,
if the reaction system consists of gas and solid phases, while the solids
existing only as pure substances, e.g., wood combustion, coal combustion, pyrolysis of solids.
1.2. Without selecting the calculation option "Turbulent System (High Entropy)"
Calculation is terminated when the sum of the fugacity and activity of the
substances in a phase corresponds to the system pressure.
The sum of the chemical activity of substances for each phase:
Σai
≈ 1
In several case, the sum of the activity or fugacity in a phase can not
correspond to the system pressure,
In this case, the application may display a message, or the reason can be seen
from the calculation results.
1.3. In the both case of the calculation options
for each possible reaction in system:
as e.g.: a A + b B = c C + d D
the following equations apply regardless of the selected calculation option
K=( [A]a . [B]b)/( [C]c . [D]d) = exp(-ΔG°/R T)
[A], [B], [C], [D]: activity or fugacity of the substances in the equilibrium state
ΔG° = c G°C + d G°D - a G°A
- b G°B
G°i(T,P) [J/mol]: the molar free
energy of pure substance i at the temperature T
and
c GC + d GD
= a GA
+ b GB
Gi(T,P) = G°i(T,P)
+ R T ln ai
Gi(T,P) [J/mol]: the molar free energy of substance i in the equilibrium state.
1.4. Turbulent Reactors
| In the thermodynamic modelling of high-temperature reactors with multiple
liquid phases, we achieve a nearly exact representation of the processes by
selecting the algorithm 'Maximum entropy'. As a result of high turbulence, liquid, solid particles and gas bubble exist in other phases for a short time (e.g. liquid metals dispersed in slag, or aerosol, metals dispersed in slag or aerosol) . It is probably not always in every phase Σ a i ≈ 1 |
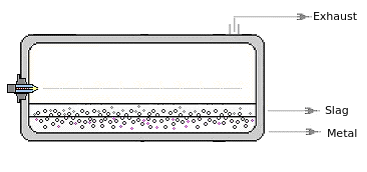 |
2. Answer to the questions whether and how a process can be modelled or represented using thermodynamic calculations.
When we intend to create a thermodynamic model of a process, some of the circumstances to be considered are:
2.1. Reactions are possible
Example, when an O2 molecule meets a CH4 molecule at 800 C, it reacts
immediately, because the ignition temperature of CH4 is exceeded.
The extent of the reaction between O2 and CH4 is determined by natural laws and
thermodynamics.
O2 also reacts immediately with C, CO, CH3OH, CH4 and several other substances
at 800 C.
The cooling process of exhaust gases below 500 °C is difficult to calculate,
even in an optimized reactor,
since the ignition temperature of several substances is fall below, including CH4,
H2 and CO.
If a reaction cannot occur, thermodynamic calculations are only of limited use.
2.2. Flows, turbulence and geometry, sufficient mixing is ensured
It depends on how fast an O2 molecule hits a CH4 molecule.
An important factor is the turbulence (Reynolds Number) and the geometry of the
reactor
2.3. The assumed temperature is approximately everywhere in the reactor.
In a reactor with an optimized flow and an approximately uniform local
temperature, the product composition can be calculated thermodynamically with
sufficient accuracy.
2.4. Liquid metal oxides are often unaffected by CO(g), CH4 (g), or
H2 (g).
The reason why liquid metal oxides do not react with substances in the gaseous
or solid state is often due, among other things, to the surface tension or the
activity coefficient of MeOx(l) at the surface.
Based on measurements, the activity coefficients of MeOx(l) can be determined
using thermodynamic calculations.
2.5 Carbon in the pure solid phase does not react directly with liquid
metal oxides (FeO in a blast furnace).
Carbon only reacts with FeO(l) when carbon is dissolved in the liquid phase with
the addition of CaO and SiO2.
The chemical potential of dissolved carbon in slags can be determined based on
the chemical potential of CO, CO2 or O2 in the gas phase.
2.6. Thermodynamic calculations are helpful in determining which product
composition can be formed
when, for example, a given amount of O2 reacts with the input material.
By comparing the calculated and measured composition of the products, we
determine which O2 fraction reacts in the reactor and what the proportion of
leak air is.
2.7. Reliable heat and mass balance using thermodynamic calculations
The heat and mass balance of a process requires substantial composition of
the input materials and products.
Often we only have the elementary composition of the input materials, residues
from other processes and/or natural ores.
Thermodynamic calculations make it possible to determine the substantial
composition of a material.
How such calculations are
carried out reliably is explained in DeterminationOfSubstantialComposition.pdf.
2.8. Process analysis, and incident prevention
Thermodynamic calculations enable reliable process analysis.
We obtain information about the behaviour of environmentally relevant substances
and elements (e.g. As, Cd, Cl, Hg, Sb, Tl )depending on the process conditions
and the accompanying substances (e.g. BaO, MgO, SiO, FeO) in the input material.
We can avoid multiple incidents caused by unwanted substances in a phase, e.g: Substances containing the elements Cl, Cd, Hg, S, Tl in the exhaust gas or Substances containing the elements Tl, S in a metal phase